German researchers have developed a new type of molecular sieve based on tiny capsules featuring the metal molybdenum. They say that their new materials can be tuned to trap different molecules depending on their size and shape and so could be useful as nanoscale filters, catalysts, or sensor materials.
Natural and synthetic porous minerals have been used by chemists for years as molecules sieves and filtration materials, as catalysts and as the molecular recognition units for sensors. The ability of these compounds, which includes the zeolites, with their pores and channels to trap molecules of specific shape and size has been well researched. However, Achim Mueller and colleagues at Bielefeld University in Germany believe discrete structures, that carry none of the mineral scaffolding of the zeolites and their chemical cousins could be much more useful. They have now built a well-defined, discrete, or molecular, compound that is essentially a naked pore.
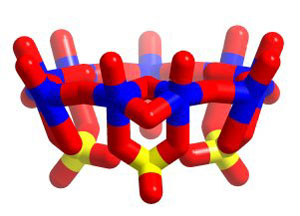
(Credit: Wiley)
These naked pores are based on a spherical skeleton composed of several molybdenum atoms and interconnecting chemical bridges. The molecule has entry points for smaller chemical species, such as cations. By fine-tuning the chemical groups around the sphere and in particular the entry points, the team can make their naked pore have affinity for specific chemicals, which can enter and become trapped inside. The skeleton – with chemical structure (pent)12(linker)30.{(Mo)Mo5O21(H2O)6}12{Mo2O4(ligand)}30 – can be crystallised to make solids that have what the team describes as sizeable pores and channels with finely sculpturable interiors. Most importantly, the chemical functionality of the channels and the size and charge of the individual capsules can be extensively varied.
The researchers have now shown that they can position different chemicals at well-defined positions above, below, and inside the channels. This situation allows us to study, in principle, new types of molecular transport phenomena, including osmotic-type ones, on the nanoscale, and shows properties of a nano-ion chromatograph, says Mueller.
Such a molecular device could be used to separate different chemicals in much the same way as a laboratory-scale chromatograph can but on a much smaller scale and with minute quantities of materials. Small quantities of cations can be separated according to their size and charge, Mueller told Spotlight, larger species cannot enter, but get trapped in the pores.
Our capsules can be considered as nanoscale laboratories, enthuses Mueller, with a variety of functionalities that enable the positioning of substrates and cations under controlled conditions. He adds that cation trapping in the pores and channels is possible in steps so that each cation trapped subsequently is affected by the decreased negative charge on the system induced by its predecessors. Such control is, Mueller says, one of the hallmarks of nanotechnology. Further on, there is the possibility of obtaining information about electrolytes under confined conditions, which is very important in biological systems, he adds.
Further reading
Angew. Chem. Int. Ed. 2003, 42, 5039-5044
http://dx.doi.org/10.1002/anie.200352358
DOI: 10.1002/anie.200352358
Suggested searches
Molecular sieves
Molybdenum elements
Nanotechnology