A common problem with portable electronic devices is that their
rechargeable lithium batteries deliver power for only a short time, and
lose their ability to be fully recharged as the battery gets older. Now,
Italian chemists have added tin and sulfur to the rechargeable recipe to
overcome these problems in next generation batteries.
Bruno Scrosati and Jusef Hassoun of the University of Rome point out
that theoretically at least, lithium-sulfur batteries would be the
energy source of choice. They would, after all, have much higher energy
density than lithium-ion batteries. However, the electrodes in such
batteries slowly dissolve and the lithium metal forms branching, or
dendritic, deposits that lead to electrical short circuits. Commercial
“lithium” batteries contain graphite through which lithium ions can
diffuse, which means no disintegrating lithium metal electrodes, but
lower energy density.
The Italian team has combined the advantages of lithium metal with the
longevity of lithium ion by developing a new type of lithium-metal-free
cell that has a negative electrode composed of a carbon/lithium sulfide
composite. The battery’s charge carrying electrolyte solution is
replaced by a lithium-ion-containing liquid enclosed in a polymer gel
membrane. The polymer protects the electrodes from corrosion. For the
anode (positive electrode), Scrosati and Hassoun chose nanoscopic tin
particles embedded in a protective carbon matrix.
The electricity generation process involves the lithium sulfide cathode
splitting into elemental sulfur and lithium ions, which releases
electrons. The lithium ions migrate through the electrolyte membrane to
the anode, where they take up electrons to become uncharged lithium
atoms, which are then bound into an alloy by the tin nanoparticles. The
process is reversible by applying a current (from the mains supply) in
the opposite direction, so that the battery can be charged repeatedly.
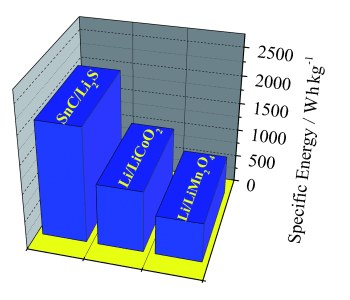
The new battery surpasses all previous attempts at a lithium-metal-free
battery with its specific energy of about 1100 Watt-hours per kilogram.
Such a high energy could not only be useful for portable music players
and mobile phones but is substantial enough for electric vehicles.
Links
Angew Chem Int Edn, 2010, in press
Scrosati