Bacteria could be the key to improving metal catalysts for the chemical industry, according to research in Germany. Scientists from the Forschungszentrum Rossendorf in Dresden have exploited the survival skills of bacteria that live in uranium mining waste to make tiny clusters of the precious metal palladium. These tiny bullets, just a few billionths of a millimetre across, are much better catalysts than normal palladium, which is used in speeding up chemical reactions and in a car’s catalytic converter
The bacterium, Bacillus sphaericus JG-A12 has a protective protein layer that allows it to survive in the extreme environment of a uranium mining waste pile. This protein layer comprises a grid of nanoscopic pockets of identical size that can trap toxic metal ions and prevent them from harming the bacterium by converting them into tiny clumps of insoluble metal.
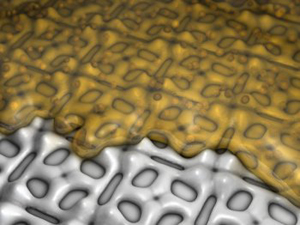
A matrix of nanoclusters made from palladium (Credit: Pollmann et al/Forschungszentrum Rossendorf)
Katrin Pollmann and her FZR colleagues reasoned that if they applied a dissolved metal of interest, such as palladium, to the protein layer the metal might form a tiny cluster of the metal in each pocket.
Within the pores of the protein layer, palladium ions are transformed into the noble metal palladium by hydrogen. The resulting nanoclusters of metallic palladium, composed of just 50 to 80 atoms, are regularly arranged on the surface layer. This combined metal-protein layer has some unusual physical and chemical properties. Because the metal stabilizes the protein and vice versa the protein layer does not break down at higher temperatures or even in acid. It is the enormous surface area to volume ratio that makes the nanoclusters interesting to chemists. The larger the surface area of a metal catalyst the more exposed metal atoms there are to speed up a chemical reaction. A palladium nanocatalyst could accelerate chemical reactions even at low temperatures.
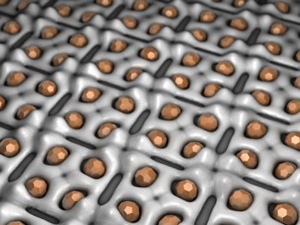
3D schematic of the bonding of the metallic salt on the protein layer (Credit: Pollmann et al/ Forschungszentrum Rossendorf)
The FZR team hopes to take their discovery a step further, however. They are now investigating whether or not they can make a gold nanocatalyst. They also hope to engineer Bacillus sphaericus JG-A12 to manipulate its protein layer so that it could make different types of metal cluster. These designer nanoclusters might have applications in optoelectronics, or as catalysts for specific reactions, as well as new physical properties researchers have so far not seen.
Further reading
Biophys J, 2006, in press;
http://www.biophysj.org/
Rossendorf home page
http://www.fzd.de/pls/rois/Cms?pNid=0
Suggested searches
catalysts
catalysis
palladium